For many of us, heat styling has become an essential part of our daily routine. It keeps our hair from looking like a giant frizzy mess, but without the right protection, it can actually cause more harm than good.
Thank you for reading this post, don't forget to subscribe!
Think of heat protectants as sunscreen for the hair. Much like our skin, the hair is prone to damage caused by heat, wind, and pollution. These external aggressors, particularly extreme heat, dehydrate and deprive our locks the nutrition they need to stay healthy. They inflict various levels of damage, from the outermost to the innermost layer of the hair. This is where heat protectants come in handy.
To understand the science behind heat protectants, it’s important to know the different types of damage heat styling can do to the hair.
4 Types of Damage Caused by Heat Styling
1. Dehydration
When heat is applied on the hair, moisture evaporates from the inside. This disrupts the intermolecular interactions between lipids and proteins in each hair strand, making it more prone to frizz and breakage. After all, lipids are our natural environmental shield barrier that protects the hair from cuticle damage and protein/moisture loss.
2. Hair Fadage
High temperatures cause pigments to oxidize rapidly. Thus, people who use heat styling tools often will notice their hair fading quicker than expected, leaving hair with that brassy warmth as the underlying pigment is exposed.
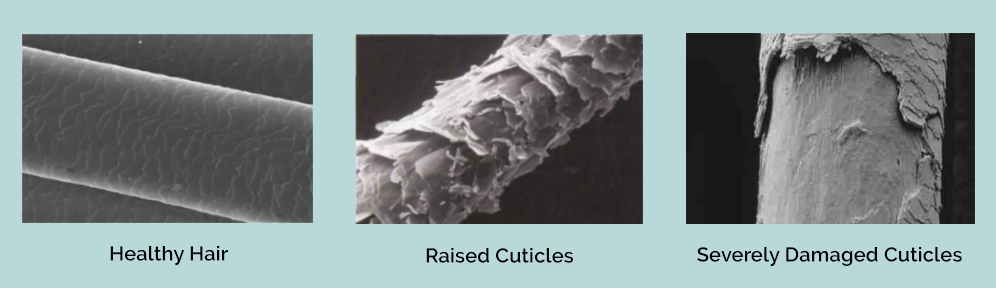
3. Cuticle Damage
Our hair is composed of tightly packed down cuticle scales which give the hair that healthy sheen look. When the hair is dehydrated from over-styling, the cuticles start to rise and become tattered when not treated immediately. This results in the hair looking dull, coarse, and frizzy.
4. Protein Breakdown
Each strand is composed of keratinized proteins which give the hair its tensile strength and elasticity. Frequent thermal styling softens those internal hair proteins, causing them to break down and eventually weaken the hair’s structure.
How do heat protectants work?
Heat protectants form a protective barrier on the hair that buffers heat conduction and distributes heat evenly, preventing local overheating. Most products are also formulated with ingredients that have been scientifically proven to reduce heat damage while providing moisturizing effects. One of them is PVP/DMAPA acrylates copolymer.
PVP/DMAPA acrylates copolymer is a cationic polymer widely used in hair styling products for the protection it provides. This ingredient has a rating of “1” EWG Skin Deep’s Cosmetic Database.
In 1998, PVP/DMAPA acrylates copolymer was found to help suppress surface damage by as much as 20%.1 It coats the hair fiber with a thin layer that serves a dual purpose: prevents moisture from escaping and slows the transfer of heat to the hair.


When combined with nourishing oils like Sunflower Oil, also a known natural heat protectant due to its high smoking point, it makes the hair appear shinier and smoother. This is in part due to the conditioning benefits provided by Sunflower Oil which keeps the cuticles from rising. As a result, the hair stays hydrated, and its color pigments stay intact (as illustrated by the Thermal Stress Test we conducted).

You can find these two ingredients, along with several other biodynamic-organic hair-strengthening ingredients, in Oway Thermal Stress Protector.
It’s important to note, however, that heat protectants cannot entirely protect the hair from heat damage. They just serve as a buffer between the appliance and the hair, and can only do so much. We still highly recommend minimizing heat styling. If this is not on option for you and your clients, applying an organic thermal protection product should definitely be part of your regular routine.
Source:
1 McMullen, R and Jachowicz, J. (1998). Thermal degradation of hair. II. Effect of selected polymers and surfactants. Retrieved from http://journal.scconline.org/abstracts/cc1998/cc049n04/p00245-p00256.html